Staying on top of all the latest food regulations and updated requirements for each market is sometimes exhausting. But not complying with regulations can be dangerous for customers and costly for your company. In the worst cases, it can get you in hot water for false advertising.
To help keep you up to date with the most important food labeling requirements in Europe and the United States, I’ve summarized all the crucial information in this guide.
So let’s begin with the EU regulations for packaged food.
Food labeling in the EU
The European Union instructs everyone who sells food and beverages to make information on packaging accurate and easy to see and understand. It also shouldn’t in any way be misleading or easy to remove.
Here are the five labeling requirements all food packaging needs to follow:
- Mandatory information for prepacked foods (more on this in a second)
- Ingredients list
- Quantity of certain ingredients
- Allergen information
- Labeling size guidelines
Mandatory information for prepacked foods
To make it as transparent and easy as possible for everyone to understand the requirements, the EU made a list of mandatory information for all packaged food labels.
Here’s what’s on the list for most packaged foods:
- Name of the food
- Ingredient list (including any additives)
- Allergen information
- Net quantity of the whole packaging, including quantities of each ingredient
- Best-use dates and the country of origin
- Name and address of the food business operator
- Instructions for use and storage conditions
- Alcohol level for drinks with more than 1.2% of alcohol
- Nutrition declaration containing energy and nutrient content information
Ingredients list
When it comes to ingredients, you’ll need to list all the ingredients that your product contains. There are only two things to keep in mind when listing your ingredients. Firstly, make sure to title that section with a heading containing the word “Ingredients”.
Then you can list your ingredients and dietary supplements in descending order of weight, being sure to use their legal names.
But, as always there are some exceptions to the rule. To find out which foods don’t require ingredients lists, and which ingredients don’t have to be reported in the list, I suggest you visit Article 19 and Article 20 of the Official Journal of The European Union.

Quantity of certain ingredients
There are a couple of specifics when it comes to labeling the quantity of certain ingredients. Firstly, you have to name the quantities of each ingredient that appears in the name of your product. For example, if you’re selling an orange juice, you have to express the exact quantity of oranges.
Then, if you emphasized a certain ingredient on the labeling in words, pictures or graphics you have to mention the quantity of those ingredients as well. For example, if you’re selling a chocolate bar with extra walnuts (and you emphasized that on the packaging), you have to note the percentage of walnuts in each pack.
Finally, you should also mention quantities of every essential ingredient that characterizes the food to distinguish it from other foods.
Allergen information
This instruction is pretty straightforward. To avoid causing any danger to your customers, you have to distinguish all allergens in your product. You can do it by writing it in a different font, letter size or background color.
In case your packaging doesn’t contain an ingredients list, you have to write the word “Contains” followed by the list of allergens.
Labeling size guidelines
The last important aspect of your packaging is the size of its label. The EU prescribes that mandatory information on each product has to be printed using a font with a minimum height of 1.2mm.
But, if the largest surface area of packaging is less than 80cm², you can use a minimum height of 0.9mm.
Deliver compliant content with confidence
Set up a consistent and compliant marketing review process with Filestage.
Food labeling in the US according to FDA
The Food and Drug Administration (or FDA for short) is the federal food agency of the Department of Health and Human Services in the United States.
The goal of the FDA is to protect the public health by making sure all drugs, biological products, medical devices, food supply, and cosmetics are safe for customers.
Here’s what each food packaging needs to contain according to the FDA:
- General food labeling requirements
- Name of food
- Net quantity of contents statement
- Ingredients list
- Nutrition labeling
- Claims
General food labeling requirements
All packaged foods sold in the US need to present food information on the packaging. And the FDA gives you detailed instructions on where, when, and how to present food information on your food label.
To make their requirements more clear, FDA defined them in terms of Principal display packaging (PDP) and alternate PDP. PDP is the part of your packaging that’s most likely seen by customers and alternate PDP are all other sides of your packaging.
Here’s what general food labeling requirements prescribe:
- There are two ways to present information on your food label – either on the PDP or on the information panel (positioned on the right side of the principal display panel).
- You need to present the statement of identity, or name of the food, and the net quantity statement, or amount of product on PDP or the alternate PDP.
- Your packaging needs to contain an information panel label. This should include the name and address of the manufacturer, packer or distributor, the ingredient list, nutrition labeling and any required allergy labeling.
- You should use a print or type size that is prominent and easy to read. More specifically, letters need to be at least one sixteenth (1/16) inch in height based on the lower case letter “o”.
- You’re not allowed to invert the material of your label and place important information inside it.
- Your packaging should contain the name and address of the manufacturer, packer or distributor, street address, city or town, state, and ZIP code.
Name of food
In the “Name of food” section of the FDA’s Food Labeling Guide, you can find even the slightest details regarding the naming conventions of your product.
From everything about how to name your product and where to place the name on the package, to all about the restrictions on your artwork.
For example, if you’re selling a chunk of sliced cheese, you’ll have to provide a modified statement of identity where you describe the exact form of food contained in your package.
Or, if you’re selling a new food that resembles a traditional food and is a substitute for it (I’m looking at you, plant-based milk). You must label your milk as an imitation if it contains less protein or a lesser amount of any essential vitamin or mineral.
The Guide also contains a whole section about the name of juice products. If you need a more detailed look, make sure to visit pages 8 to 13 of the Food Labeling Guide.
Net quantity of contents statement
When it comes to the quantity of your package, the FDA requires you to express it in weight, measure, or a numeric count. Logically, if your product is liquid, you are required to express its weight in a fluid measure.
Plus, based on the Food Labeling Guide, you have to express the weight of your product in both metric (grams, kilograms, milliliters, liters) and US Customary System (ounces, pounds, fluid ounces) terms.
If you’d like to find out more details about the principal display panel, specific sizing of your product, or how to express different weights within your product packaging, I recommend visiting pages 14 to 16 of the Guide.
Ingredients list
The Food Labeling Guide says that ingredients should be listed from high to low in order of weight or volume.
Here is some other valuable information about the ingredient list:
- The ingredient list must be placed on the same label panel as the name and address of the manufacturer, packer, or distributor
- Use a type size that is at least 1/16 inch in height, prominent, and easy to read in your ingredients list
- All information you need about allergen labeling
- How to treat ingredients in “trace” and when to report them
- How to declare spices, natural flavors, or chemical preservatives in your ingredients list
- Everything you need to know about reporting colors related to your ingredients
Nutrition labeling
According to the FDA, most foods need to have nutrition labels as part of the food packaging. Nutrition labels help users choose between different products, giving everyone a way to keep track of the amount of foods they’re eating, including percentages of fat, salt, and added sugars.
Since various products with different types of packages have distinct ways of reporting all the nutrition facts, the FDA offers a long list of regulations to cover all cases and formats. That’s why you’ll find all the specifics around different labeling formats for different products in this section of the Guide.
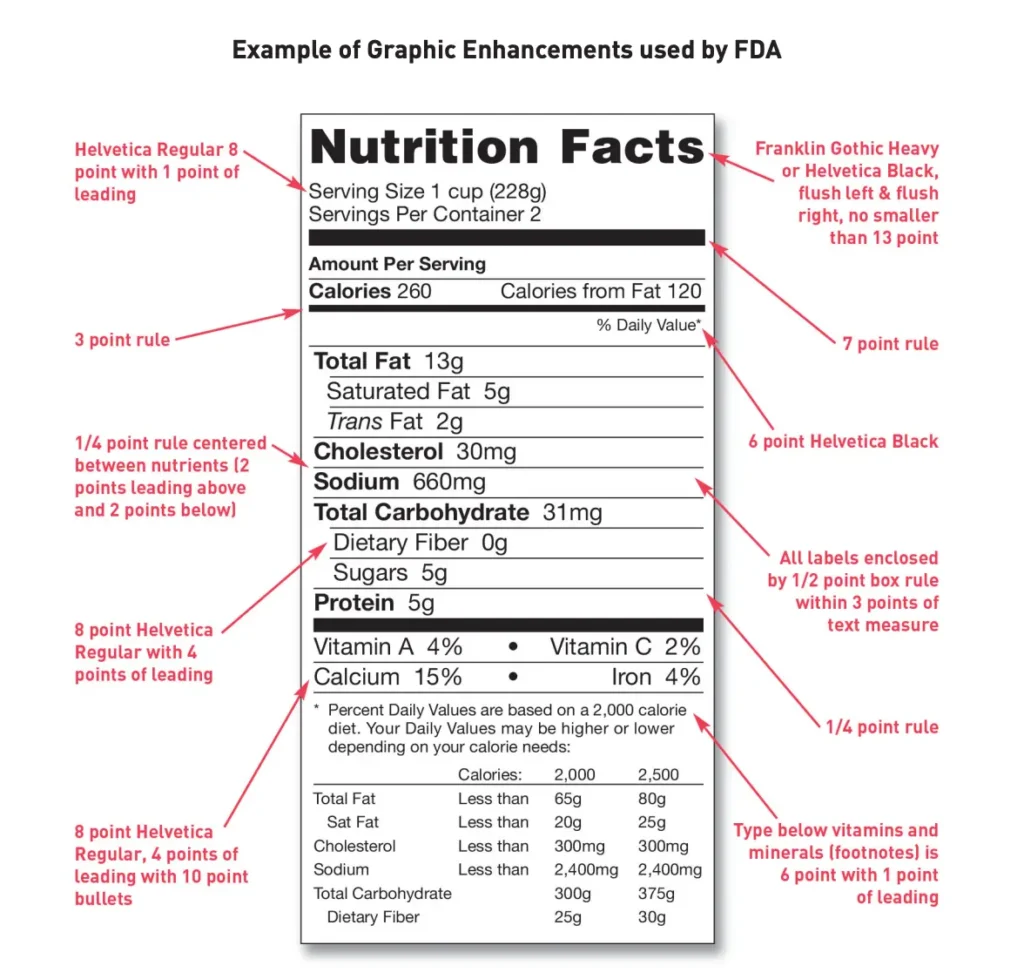
Source: fda.gov
Claims
The last section of the Food Labeling Guide is reserved for claims. To ensure food safety and compliance with relevant laws and regulations, all food labels need to contain certain claims.
Here are the three types of claims that the FDA defines for food packaging:
- Nutrient content claims – Claims that characterize the level of a nutrient in the food (for example, you can say “low fat,” “high in oat bran,” or “contains 100 calories”). Within this section, you’ll also find specific information about different disclosure statements about your product.
- Health claims – Any claim on the label that makes explicit and implicit references about any substances being connected to a disease. In this section, you’ll find out everything you need to know about reporting a connection between your product and potential health risks.
- Structure/function claims – Claims that describe the role of a nutrient in affecting the normal function of the human body. For example, claims on milk packaging about how “calcium builds strong bones.” In this section of the guide you can find detailed information on when and in what circumstances you can make those claims.
Maintain food labeling compliance with Filestage
Maintaining food labeling compliance is a difficult job. Besides staying on top of the most recent regulations, you also need to update, print, and distribute new packages across different markets whenever a new regulation appears. This is when the process can quickly become extremely time consuming.
Luckily, with a specialized packaging approval tool like Filestage, you’ll be able to streamline your packaging and artwork approval process and make sure all updates get to the market in time. Want to see it for yourself? Try Filestage for free for 7 days.