The pharmaceuticals market is forecast to reach $1,156 billion in 2024. But despite sizable budgets, marketers in the industry still face challenges.
Well, one major problem, to be exact.
40% of pharmaceutical marketers cite regulatory compliance as their biggest marketing challenge. These strict regulations complicate pharma content approval, slowing down the review process.
And as GlaxoSmithKline discovered in 2021, failure to comply could be a billion-dollar mistake. More on that later.
A structured review and approval process is the best way to keep up with high content demands in a regulated industry like pharma. And that’s what I’ll be digging into today.
I break down the hurdles pharma marketers face in the content creation process and four strategies to improve the approval process.
Content approval is a major challenge in the pharmaceutical industry
We spoke to Christa Carroll, Managing Partner at Outlook Marketing Services, about the challenges pharma companies face.
As a marketing agency, the team works with companies that sell products and services to pharmaceutical manufacturers.
“Based on our experience, securing content approval can be difficult for any company, but it’s even more challenging for pharmaceutical (and healthcare and medtech) companies – and rightly so.”
She goes on to explain, “They face tremendous risk when discussing their drugs, patient information (think safety), services, distribution methods, and production processes. Any information shared has to be 100% accurate, as they can be penalized for misinformation (regulatory fines, impact to the brand, etc.)”
These challenges highlight the importance of having a robust workflow for creating and reviewing marketing content.
Manage every approval in one place
Request approval without sending a single email with Filestage.
How to optimize your review and approval process in four steps
Research shows the pharma industry takes up to 40 days to complete the content review and approval process. As content demands grow, pharmaceutical companies need ways to cut review time without compromising accuracy.
If this resonates with you, here’s how to streamline content approval workflows in four steps.
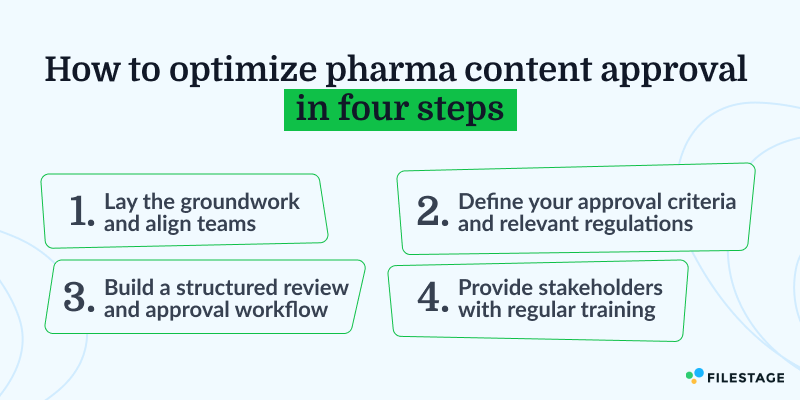
Step one – Lay the groundwork
An airtight internal review and proof approval process starts with solid foundations. That means aligning teams on content priorities, goals, and processes.
You’ll also need to identify all stakeholders involved in the content creation, review, and approval process. For instance, medical writers, marketing, legal, and so on.
Clearly defined roles and responsibilities for each stakeholder and team member will also make the next steps in the process a whole lot easier.
Step two – Define approval criteria
Meeting medical, legal, and regulatory requirements involves a deep understanding of the laws in different markets and regions.
Your first step in this process is identifying the relevant regulations you need to comply with, like FDA guidelines or other local standards.
Once you’ve done this, you can set clear criteria for your content approval process.
Creating predefined checklists for different content types can help keep everyone on the same page.
Of course, all content also needs to align with your brand style guide and messaging.
Step three – Build a structured content review workflow
Building a custom review and approval workflow is the next step in the “work smarter, not harder” process.
A creative review and approval platform is the best way to do this. With this tool, you can map the content lifecycle from ideation to final approval.
You can also automate time-consuming manual tasks, get project-level visibility, and manage deadlines for each stage of the review process.
Streamlining this workflow facilitates seamless collaboration between marketing teams, legal, stakeholders, and even regulatory departments. You also have a single system of record in case issues arise.
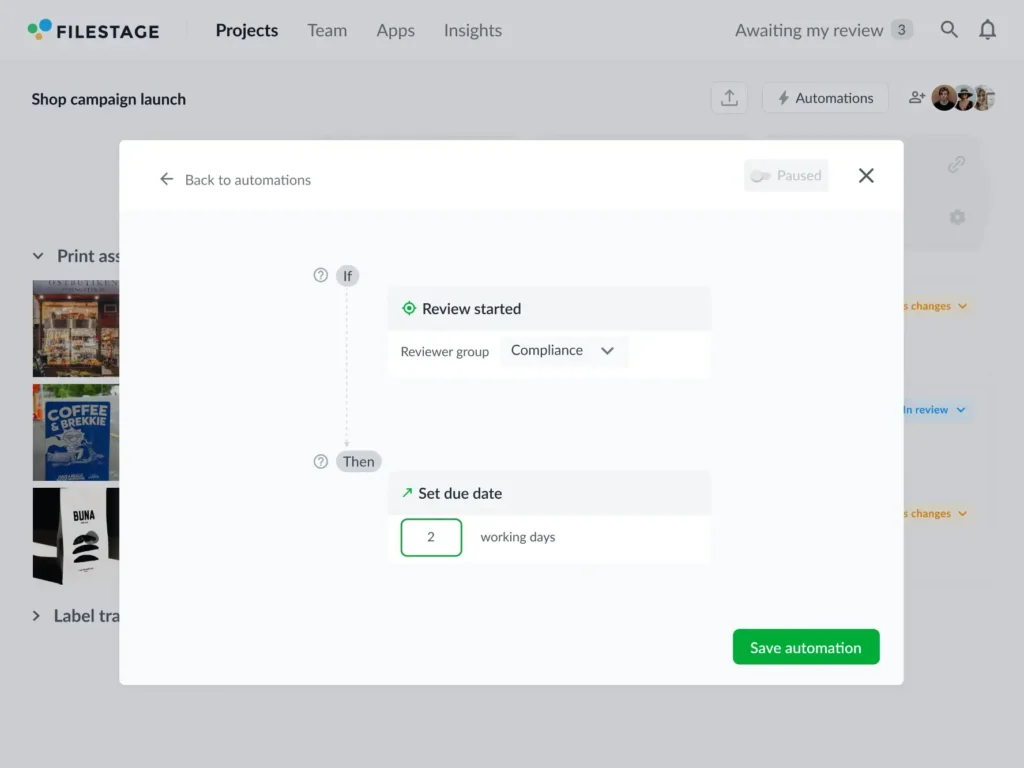
Another big plus with creative review platforms is that you can set up custom reviewer groups. That means the right stakeholders enter the review process at the right time to reduce duplicated work and time wasted.
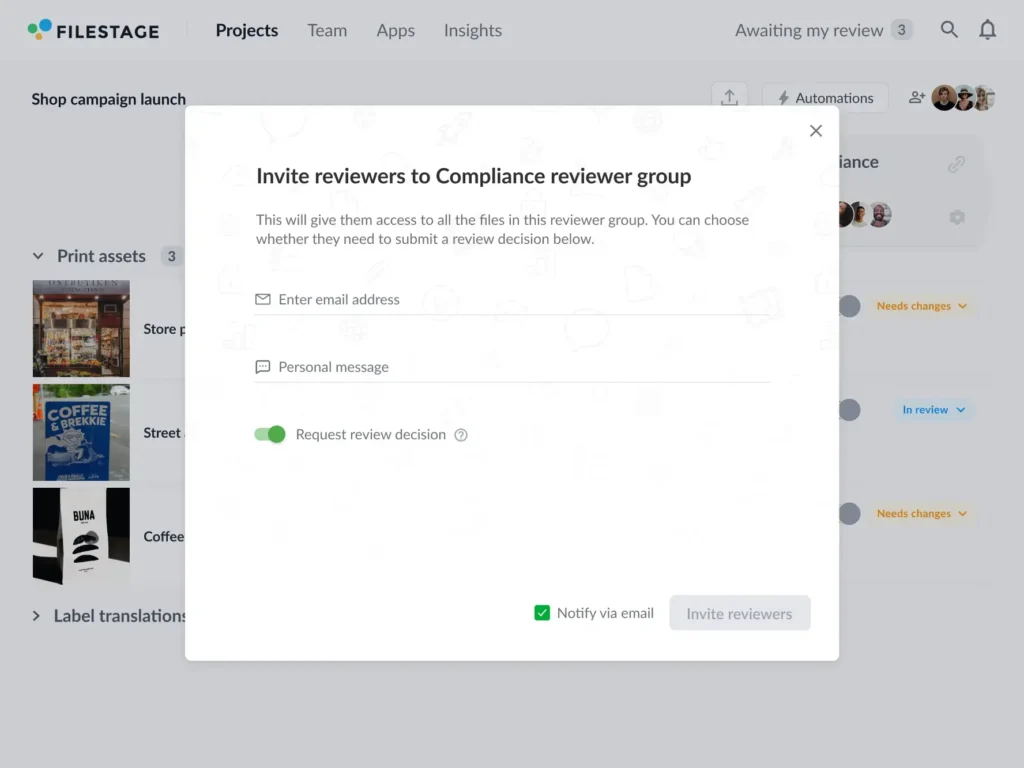
It also means you can choose between a linear process or setting up parallel reviews from different departments to save time and avoid costly holdups.
As we saw earlier, getting content approval is a slow process in the pharmaceutical industry.
According to Georgia Gayle, Head of Commercial Promotional Review and Marketing Operations (US/Global and Japan) at Alexion Pharmaceuticals, artificial intelligence is the answer.
AI-driven review and approval platforms can automate parts of the process and serve as an extra pair of eyes.
For example, Filestage has built-in AI tools that help reduce approval timelines while maintaining exceptional quality control.
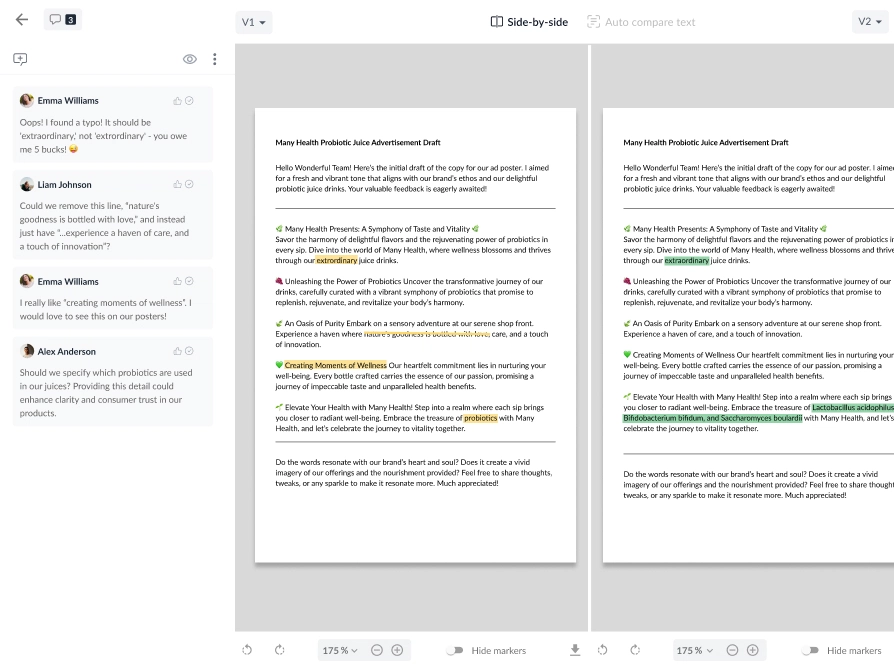
Here’s how it can support you:
- Create a unified platform for all content assets and feedback so nothing slips through the cracks
- AI-powered version control so you can visualize content changes (our AI flags inconsistencies the human eye might miss)
- Leave pinpoint-precision feedback on visual content like videos and infographics where errors are often missed
- Set up custom reviewer groups to get the right stakeholders involved at the right time
- Automate time-consuming tasks like deadline reminders to get your promotional materials to market faster
Step four – Provide all key stakeholders with ongoing training
With big stakes and fast-moving changes, pharma companies need to keep their finger well and truly on the pulse.
Every stakeholder in your pharma content approval process should have regular training to keep up with industry best practices and regulatory updates.
On top of regular consultations with legal and regulatory bodies, consider setting up Google alerts and subscribing to relevant newsletters.
Last but not least, user feedback is an often overlooked tool for optimizing your review and approval workflow. Your workflow is a living, breathing thing that requires regular upkeep.
So, gather data from users and track key metrics (like speed to market) to gauge how effective your current workflow is.
💡 Additional reading: How to create and manage a content approval workflow in 2024
Standard pharma content compliance regulations
Everything from social media posts to promotional content falls under strict regulatory scrutiny.
But here are some of the most common content compliance checks in pharma.
- Product labeling must include clear and accurate information about the product, its use, dosage, and side effects so consumers can make informed decisions
- Packaging inserts must provide comprehensive information
- Clinical trial data must be transparent, accurate, and accessible for review
- Packaging design must be fit for purpose
In a 2022 interview with PharmExec.com, the Vice President of Indegene, Sameer Lal, shared insights into the challenges when reviewing promotional content.
“The bottom line is that even as the amount of content being submitted for review to internal MLR teams has grown 3-4 times in the past few years, the size of the review teams has not increased anywhere close to that extent.”
Not to mention the growing challenges of approving digital content, which comes in a wide variety of formats and varied data visualization.
Finally, Sameer warns that the growth in specialized agencies means not everyone is fully versed in the requirements for the MLR review process.
Consequences of non-compliant content
Holes in your pharma content approval process can be costly, to say the least. Crippling lawsuits aside, failing to meet regulatory requirements could be a life-or-death mistake for your consumers.
Here’s a closer look at the biggest potential risks.

1. Danger to public safety
Misleading claims, failing to share the risks and side effects of the product, inaccurate labeling information … any of these could endanger the public.
Pharmaceutical companies are up against strict scrutiny when their content fails to deliver transparent and accurate information.
A recent example is Purdue Pharma.
The company came under fire for fuelling the opioid crisis in the US, leading it to file for bankruptcy in 2019. The owners also had to pay a six billion dollar settlement to protect themselves from further lawsuits.
2. Damaged brand reputation
Even if your business can weather the legal and financial implications of a major lawsuit, it may never recover its public image.
Distrust surrounding the pharmaceutical industry is high, meaning any controversy or black mark against your company name could do irreparable damage to your reputation.
This is not a new issue.
A Gallup survey asked Americans to share their perceptions of different industries. The pharma industry came in dead last.
3. Fines and lawsuits
An ineffective content review and approval process can be a billion-dollar blunder.
Without a proper process, pharma companies leave themselves open to lawsuits and fines from regulatory bodies like the FDA.
Do you remember the GlaxoSmithKline LLC case from 2012?
The company pled guilty to promoting prescription drugs without accurately reporting safety information. The result was a $3 billion settlement. Ouch.
4. Operational disruptions
Issues with packaging, product safety, or labeling could lead to your business having to withdraw pharma products from the market. That’s an expensive process. On top of that, it can cause halts in production and delays in distribution.
This was the case for Cipla in 2023.
Cipla is a subsidiary of InvaGen. Just last year, it voluntarily recalled a lot of its anti-seizure drugs because of a faulty seal in the packaging. The product was later redistributed, but I imagine this was a costly design flaw.
With so much room for error, core regulatory bodies like the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) work hard.
As well as overseeing product quality, they weigh in on content creation.
For example, the FDA reviews pharmaceutical marketing materials to make sure they meet the relevant criteria and maintain the integrity of the industry. And most importantly, to protect public safety.
It examines promotional content to verify all claims are accurate, fair, and free from misleading information. Only when it finishes this rigorous review process does it approve the content.
Final thoughts
Pharma marketing poses some hefty challenges. Long approval times and stringent regulatory compliance can complicate the process and impact your content strategy. Fortunately, the advice in this guide can help combat this.
If you’re looking for an online proofing tool to optimize your pharma approval process and reduce compliance headaches, grab a free seven-day Filestage trial today.