When you think of artistic creativity, the pharmaceutical industry probably isn’t the first thing that pops up. But you’d be surprised at how much creative energy goes into designing, developing, and reviewing assets.
It’s a tightly-regulated industry with high compliance standards, so the artwork approval process in pharma needs to be efficient.
That’s what I’m breaking down in this article.
Keep reading for five best practices to make your artwork management process as streamlined and headache-free as possible.
1. Define the key stakeholders
The artwork approval process is a team effort in any industry. But especially pharma. The right group of experts makes sure your artwork meets regulatory, quality, and creative standards.
So, the first step to building a better workflow is to identify your key stakeholders. Here are some teams that should be involved in the process:
- Marketing – in charge of the initial design concept and messaging
- Regulatory affairs – makes sure the artwork meets government standards
- Packaging development – turns the concept into artwork
- Quality assurance – performs quality checks to look for accuracy and artwork design flaws
- External printing partners – produce the final printed packaging materials once the artwork is approved
Once you’ve identified your stakeholders, it’s time to decide when to involve them in the approval process. Ideally, you should segment your reviewers to make sure the right people approve your artwork at the right stage of the process.
Using pharmaceutical review software like Filestage can help you do this.
Less time chasing feedback, more time on creative strategy
Online proofing software can cut review cycles by 30%, freeing up time for high-stakes work. Read our guide to see if it’s the right solution for your team.
Filestage is designed specifically to streamline and secure the creative review process for companies in regulated industries.
Here’s how your pharmaceutical promotional reviews could look in Filestage:
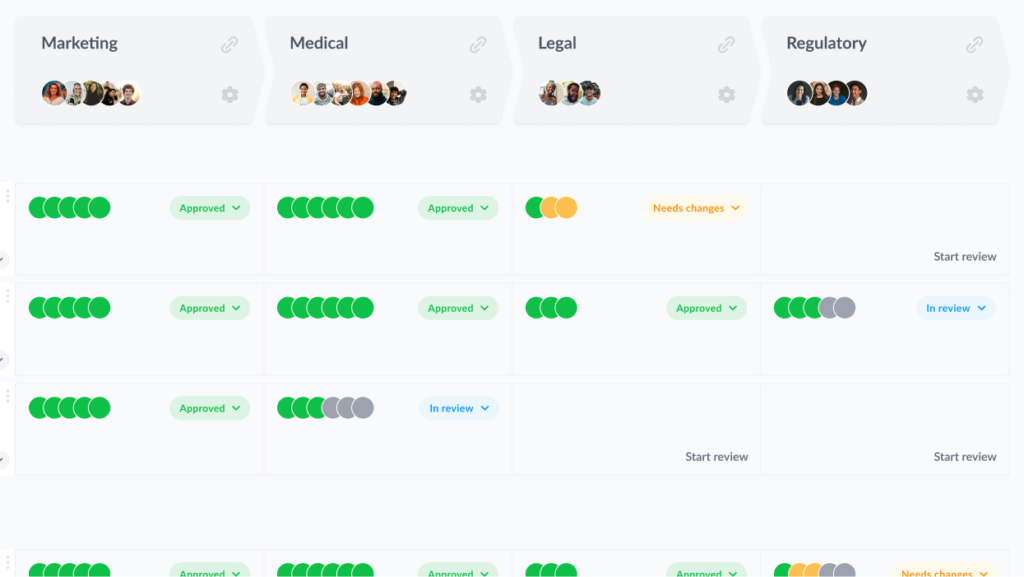
2. Standardize the artwork approval process
Data from the US Food and Drug Administration (USFDA) revealed that in a six-month period, it recorded 455 recall notices. Interestingly, 51% of those were due to mislabeling, and 13% were as a result of faulty packaging.
A standardized artwork approval process will save you a lot of time and compliance headaches. Trust me.
Here are the main benefits of designing a well-defined workflow:
- Improves consistency and cohesion in your artwork
- Reduces errors and maintains product quality
- Makes sure key review stages are never missed
- Helps you meet regulatory requirements
- Streamlines collaboration with cross-functional teams
- Helps you make sure you’re sending error-free pharmaceutical labels to print
Start by defining a standard process with clear steps and timelines.
Then, build pre-defined templates for artwork submissions to make sure you have all the key information from the start of every project.
You can automate this process with Filestage. Once you create a custom workflow, you can save it as a template and reuse it across multiple projects – from packaging to in-store advertising displays.
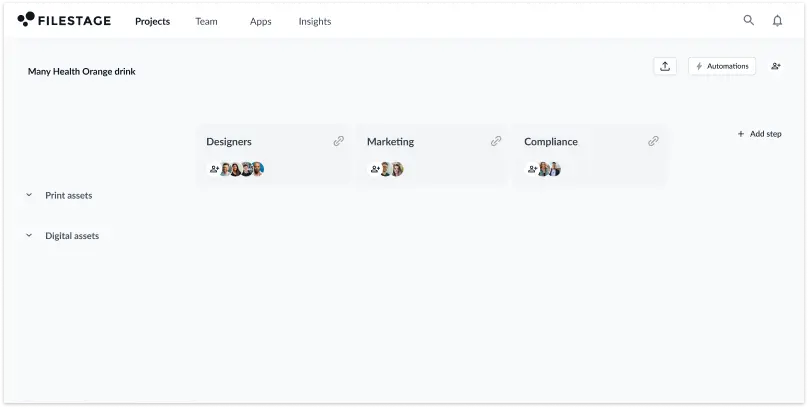
You can also automate time-consuming manual tasks like setting deadlines and moving approved versions to the next review stage, shaving up to 30% off your pharma content approval timeline.

“Filestage saves our company a huge amount of time. Instead of writing detailed instructions over email, reviewers can just click on a file to add comments in context. This makes everything faster – from giving feedback, to managing the process, to making edits before sharing the next version.”
Karina Berner, Creative Production Specialist at Sartorius
3. Understand the specific regulatory requirements
Pharma marketing compliance can be complex to understand, especially if you operate across multiple regions with different standards.
That’s why it’s crucial that you’re clear on the specific requirements from the beginning of the artwork creation process.
Integrating regulatory experts at the start gives you a better chance of spotting potential compliance issues early on. And it will make sure your brand isn’t responsible for the next big false advertising fail.
Once you have a solid understanding of the regulatory requirements, define checklists and reference materials to guide team members at every stage of the creation and review process.
It may require an initial time investment, but it can significantly cut delays and reworks in the late stages of your project.
4. Manage artwork from a centralized hub
Scattered feedback and versioning chaos are very real symptoms of an ineffective artwork approval process. They can also lead to serious compliance issues if key feedback is missed or the wrong version of your design finds its way to the market.
Fortunately, there’s a way to reduce these bottlenecks.
Managing the full artwork process in a review and approval platform like Filestage allows you to centralize all assets, feedback, and content versions.
Here’s how you can streamline artwork management with Filestage:
- Create a central repository for content assets and feedback
- Leave in-context feedback directly on packaging artwork (no more neverending email threads)
- Manage old content versions so everyone’s always working on the right iteration
- View versions and comments side by side to confirm feedback has been implemented
- Integrate Filestage with other tools in your tech stack for a seamless experience

Manage every approval in one place
Request approval without sending a single email with Filestage.
5. Measure your performance
Think of your artwork approval process as a living, breathing part of your organization. As compliance requirements continue to change quickly, you need to check in on your workflows regularly to make sure they’re still optimal. You can do this by looking for bottlenecks and identifying where you need to make improvements.
The first step is to get feedback from reviewers and stakeholders to find out where they’re having issues.
Next, look at the data.
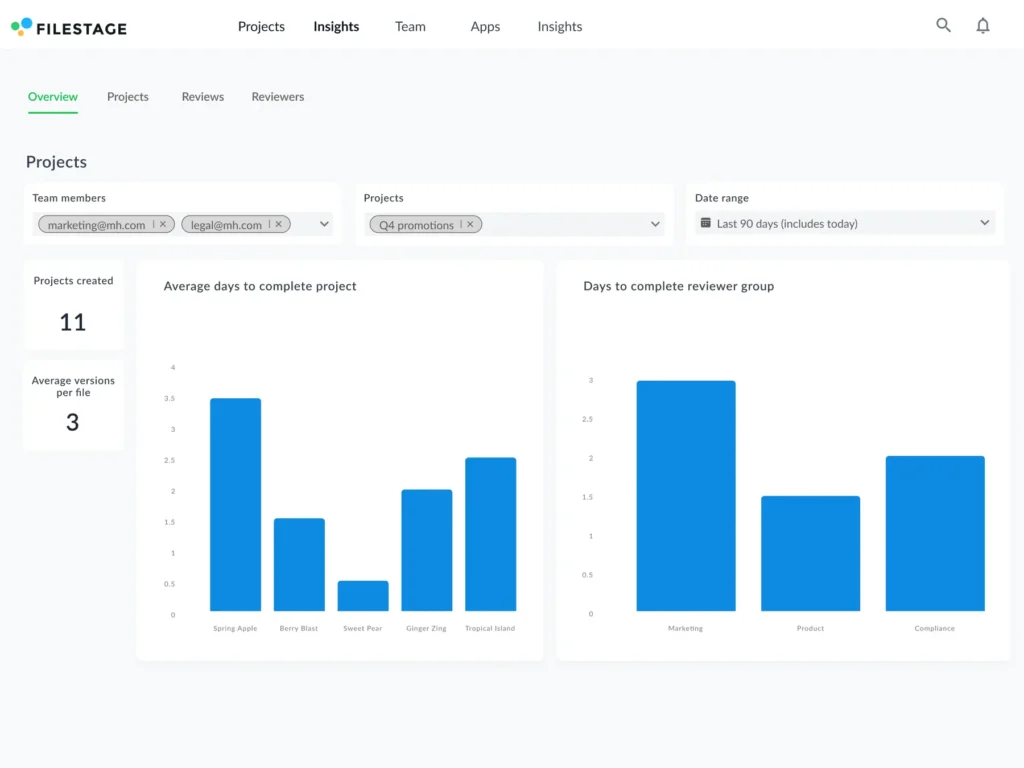
With Filestage Insights, you can quickly visualize how your artwork review process is performing and gauge your team’s productivity.
Here’s how the dashboard looks:
Why the artwork approval process in pharma matters
Pharmaceutical artwork isn’t just about aesthetics. It represents key information on medication packaging, labeling, and promotional materials. That includes details about usage, dosage, and potential side effects.
Both labeling and pharma packaging artwork play a pivotal role in safeguarding users, helping them make informed decisions about the product.
That’s why medical artwork has to meet strict regulatory requirements that ensure all the information provided is accurate and clear. Regulatory bodies must make sure that every piece of artwork (from the package insert to the label) meets industry guidelines.
With so many reviewers involved, the artwork approval process is complex and time-intensive.
Streamline artwork management with Filestage
Thanks to stringent regulatory compliance standards, quality control is a very real necessity in pharma. An effective workflow streamlines lengthy review processes, keeps all stakeholders aligned, and makes sure your packaging artwork complies with legal requirements. So you can get faster approvals.
Ready to build a better artwork management system? Get a free seven-day Filestage trial today.