In the last 20 years, pharmaceutical, biotech, and life science companies have shelled out over $12 billion to settle lawsuits. One major cause of these fines is inaccurate marketing and product labels, which is where MLR reviews come in. Here, we’ll look at what they are, who’s involved, and how software can help.
What is MLR review?
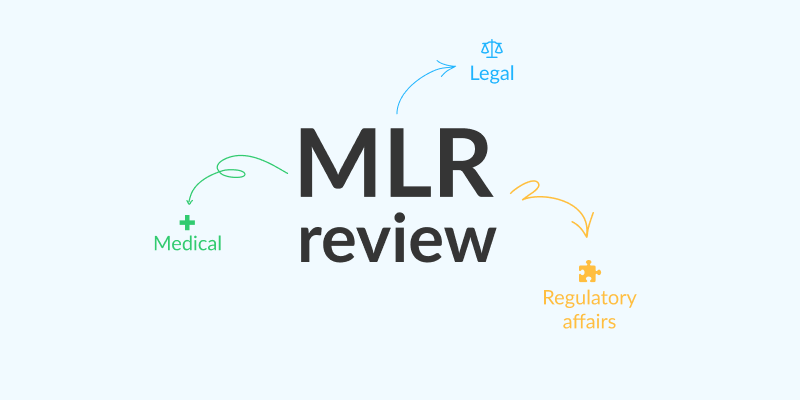
MLR review is short for medical, legal, and regulatory affairs review. It’s an essential process in healthcare marketing to make sure advertising and promotional content is accurate, compliant, and ready to be published. This includes everything from posters to designs for medical packaging.
If you work at a healthcare agency or in a pharmaceutical, biotech, or life sciences company, you probably know about the struggles you can have with the pharmaceutical promotional review process. But before we get into who’s involved and why it’s important, let’s do a little more jargon-busting.
Here are a few other terms you may need to know about:
- Med-legal review: This is the process of getting feedback and approval from medical and legal reviewers. In this process, regulatory affairs specialists are usually merged into the legal side of the med-legal review. You may also hear this referred to as the medical-legal review, which, just for fun, can also be shortened to MLR.
- CMLR review: The C in front of MLR here stands for “compliance”. If your content is being localized to different markets, it will need to be checked by a compliance specialist with local expertise. And in most cases, you’ll be asked to add a disclaimer to comply with the local area’s rules. Confusingly, the C in CMLR may also stand for “commercial”. So if in doubt, just ask!
- PRC meetings: Depending on who you talk to, PRC can stand for promotional, process, or protocol review committee (sorry if I missed any). But in all cases, the meaning is the same. It’s a very, very long meeting where marketers and MLR reviewers discuss all the latest content being created. In most companies, these are held once or twice a month.
Who’s involved in each stage of the MLR review
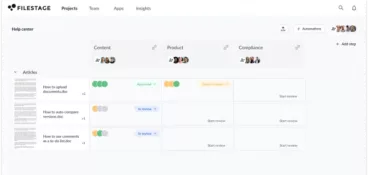
The MLR review for pharma companies involves three groups of stakeholders, each specializing in a different area. By collecting feedback and getting approval from all of these groups, you’ll be able to publish your content with confidence – and without fearing a lawsuit!
Let’s take a closer look to see what each group brings to the table.
Medical
The medical review round is all about scientific accuracy. Whether you’re creating a sales aid, implementation guide, or product brochure, your medical reviewers will check the content to make sure it’s factually accurate and in line with the latest research.
Here are a few of the roles you may see in the medical review round:
- Product Manager
- Scientific Consultant
- Medical Director
- Medical Device Manager
- Medical Manager
Legal
The legal review step makes sure nothing is communicated that could put your company at risk. That means checking your content against internal guidelines and regulations or making sure no unsubstantiated claims are made.
Here are a few roles you may see in the legal review round:
- In-house Legal
- Qualified Person
- Corporate Counsel
- Legal Manager
- Legal Director
Regulatory affairs
The regulatory affairs review is all about making sure your content is on-label. They manage a rigorous process with local authorities and produce dossiers to get drugs and devices approved. So they need to check that the marketing aligns with the pre-agreed documentation.
Here are a few roles you may see in the regulatory affairs review round:
- Regulatory Affairs Specialist
- Global Labeling Manager
- Regional Regulatory Strategist
- Regulatory Affairs Manager
- Regulatory Affairs Officer
Why the MLR review is so important
The healthcare industry is driven by innovation. From pharmaceuticals to biotech, people want to deliver the best drugs and medical devices for their patients – and they want to do it as quickly as possible.
But healthcare is also a high-risk industry, where the approval of drugs and quality and accuracy of communications can literally mean life, death, or disability.
In 1958, thalidomide was released under the brand name, Distaval. Their advertisement said: “Distaval can be given with complete safety to pregnant women and nursing mothers without adverse effect on mother or child.” It took five years for the connection between pregnant women and their children to be made, at which point it was too late.
Since the thalidomide scandal in the 1950s and 1960s, pharmaceutical companies have introduced stricter testing programs for new medicines. And they’ve also tightened the screw on the review and approval of marketing content.
Here are three reasons why the medical, legal, and regulatory (MLR) review is so important:
- Your patients’ health could be at risk
When you’re creating healthcare content, your audience is usually doctors, patients, or both. And if your content includes things like dosing instructions or details of side effects, you need to be confident that they’re 100% accurate. - Your company could be fined millions of dollars
In most industries, inaccurate or misleading marketing may get banned. But in the world of pharmaceuticals, biotech, and life sciences, you’re looking at a hefty fine that can easily exceed $100 million. - You could ruin the reputation of your brand
In this case, one plus two equals three. Your brand is in every newspaper across the country – and for all the wrong reasons. And suddenly your partners and patients want to take their business somewhere else.
And of course, there’s also the cost of reprinting packaging and marketing materials to consider. To make sure you don’t get caught out, take a look at our five best practices for artwork approval in pharma.
How software can help you manage your MLR reviews
Over the last few decades, pharmaceutical, biotech, and life science companies have been transitioning from paper-based MLR reviews to email. But it’s far from perfect. And with the recent boom in collaboration tools like Microsoft Teams and Jira, more and more companies are now switching to cloud-based solutions.
As a writer at Filestage, I have a unique insight into how our MLR review software helps healthcare companies – including Publicis Health, B. Braun, and Queisser Pharma. Let’s take a look at how Filestage has transformed their MLR reviews.
Give reviewers a more transparent experience
Before using Filestage, Publicis Health had issues with version control and MLR review management. Medical Director, Sarah Chen, says they “would end up with multiple PDFs with different feedback and no consolidation or agreement between them.”
Since using Filestage for their MLR reviews, “comments are clear and in the same place so that everyone can work more collaboratively.” On top of that, “clients really appreciate the ease of use and being able to have a clear overview of their projects.”
Speed up feedback and review rounds
Time has always been an issue for MLR reviewers. When looking into Filestage, B. Braun’s Digital Communication Specialist, Zsolt Arnodi, says he wanted to “improve the project turnaround time” and “reduce long email threads and revision loops”.
Comparing life before and after Filestage, he says “our review turnaround time has significantly improved. He also likes that his team “can easily review all kinds of content formats without any issues”, including “video, audio, and even websites”.
Create a more positive MLR review culture
In most cases, the biggest problem teams face with MLR reviews is the process’s reputation in the company. Giving clear feedback is difficult and the back and forth can quickly get messy. As Queisser’s Marketing Director, Timo Simonsen, says, “large email attachments, non-transparent feedback, and file versioning issues have pushed the feedback process via email to its limits.”
With Filestage, he says that “the accessibility of the system makes it very easy to provide feedback on creative advertising and packaging materials.” Best of all, this has “led to a positive culture change towards transparent collaboration within the company and between agencies”.
Final thoughts
To wrap up, MLR review stands for medical, legal, and regulatory affairs. It’s essential for avoiding risks to patients, lawsuits, and brand own goals.
With email, the MLR review process can be slow and frustrating. But software like Filestage gives reviewers a more transparent and positive experience.