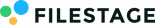The amount of content being submitted for MLR reviews has increased threefold in recent years. At the same time, pharma companies are producing content for a wider range of digital channels and privacy laws are getting stricter.
As a result, there’s more pressure than ever to build a streamlined and effective pharmaceutical promotional review process.
That’s what I’ll be looking into today. Keep reading for four best practices on how to build an airtight review and approval process for promotional content in the pharma industry.
But first, let’s align on what the process is and who should be involved.
What is the pharmaceutical promotional review process?
The goal of the promotional review process is to make sure all materials are accurate and compliant with the market’s specific medical, legal, and regulatory requirements. This includes everything from pharmaceutical packaging to artwork for advertising.
Experts will check that all promotional materials are transparent and don’t mislead consumers about the risks and benefits of the product. That includes written, audio, and video content.
During the process, qualified reviewers check that all claims or data are backed by substantial evidence from FDA-reviewed trials.
It’s also an important tool to maintain ethical marketing practices in the highly regulated pharma industry.
According to Med Communications, this multi-phase review process also makes sure that all efficacy claims are properly balanced with accurate safety information.
“While the balance between regulatory compliance and the need for effective communication of product information in medical and promotional materials is a delicate one, it can certainly be achieved with the right review services.”
Supercharge your pharmaceutical reviews
Share, review, and approve all your content in one place with Filestage.
Who’s involved in the promotional review committee?
Thanks to stringent regulations and high standards, the MLR process requires a diverse team of reviewers with different expertise. That includes:
- Marketing team – designs and develops advertising and promotional materials
- Medical affairs team – offers scientific expertise and reviews the accuracy of the information
- Legal team – checks that promotional materials comply with legal regulations
- Regulatory affairs team – makes sure the content aligns with FDA regulations and other relevant standards
The PRC is a multi-disciplinary body that includes representatives from the MLR teams mentioned above. It discusses the findings and makes the final decision – either approving the materials or requesting revisions.
Four best practices to optimize the pharmaceutical promotional review process
The content review and approval process can take up to 40 days in the pharmaceutical industry. These delays block your drug from getting to market and prevent patients from accessing it, losing your business revenue.
The good news is that, whether you’re working on a traditional campaign or testing the latest healthcare marketing trends, there are ways to speed this process up without sacrificing quality. Here are some best practices to help you build an effective workflow.
1. Define clear roles, responsibilities, and expectations
Every reviewer in the PRC process plays a pivotal role in getting promotional materials approved in a timely and accurate manner. When reviewers are unsure about their role or what’s expected of them, they can lose focus or slow down the process.
With that in mind, establishing clear roles and responsibilities can streamline workflows and prevent unnecessary delays or confusion.
Start by breaking down the responsibilities of each MLR reviewer:
Medical reviewers
This reviewer group focuses on the scientific and clinical data provided on the product label and source materials to prevent misleading messages.
Legal reviewers
Legal reviewers hone in on specific legal concerns like false claims, product liability, copyright issues, and Anti-kickback Statute violations. They also check materials to prevent off-label promotion.
Regulatory reviewers
Regulatory reviewers check that promotional materials align with the regulatory agency-approved labeling. They make sure the materials offer a fair balance when presenting product efficacy and safety information and that all claims are supported by reliable scientific evidence.
Regulatory reviewers also oversee the submission of promotional materials to the relevant regulatory agencies like the FDA’s Office of Prescription Drugs Promotion (OPDP).
Here’s an example of how those reviewer groups should be organized during the MLR process.
A snapshot of how the promotional material medical review process could look in Filestage.
The next step is to establish specific procedures and expectations for each type of reviewer. These procedures should cover how the escalation process works when the PRC committee can’t reach a general consensus.
2. Implement a centralized digital hub for content reviews
The PRC process is slow. Really slow at times. One of the main reasons for this glacial speed is that it takes a long time to get all the PRC members in the same room to actually complete the review process.
When we couple this with scattered feedback, versioning confusion, and dispersed communication, it’s not surprising it can take over a month to get pharma content approval.
There are ways to tackle these roadblocks. Namely, committing to a digital content review and approval process.
That’s where robust pharmaceutical review software comes in.
Implementing a centralized platform to manage all pharmaceutical content (from promotional materials to packaging design) can streamline workflows and create a seamless process.
For example, Filestage is designed to help companies in regulated industries to:
- Digitize the content review and approval process
- Build a central repository for all pharmaceutical materials
- Speed up feedback cycles by leaving in-context comments directly on the visual asset
- Get project-level visibility of the content review process
- Create custom workflows to ensure the right reviewers join the process at the right stage
- Keep thorough records of all content changes and iterations
- Send error-free pharmaceutical labels to print
These features make MLR review management easy, reducing review times while maintaining the highest possible quality.
Plus, the built-in security measures offer a safer and more effective way to share feedback on sensitive content.

“Filestage is one of the only tools that lets our marketing team and agency partners collaborate in one platform. Our internal team sets up the approval workflow, then the agency can upload their content directly to kickstart the review process. This makes sure everything goes through the necessary product managers and technical experts before it goes out the door, no matter whether it was created in-house or externally.”
Karina Berner, Creative Production Specialist at Sartorius:
3. Automate workflows to streamline the review process
There’s another pretty sizable advantage of moving your review process to an online proofing tool – automation. Repetitive manual tasks can grind your content review workflow to a halt, causing production delays and increasing the risk of human error.
Automation solves these issues.
Here are some tasks you can automate in Filestage for faster, more accurate reviews:
- Assigning due dates and sending reminders
- Updating file statuses (based on stakeholders’ review decisions)
- Sharing the most up-to-date versions with the next reviewer group once approved
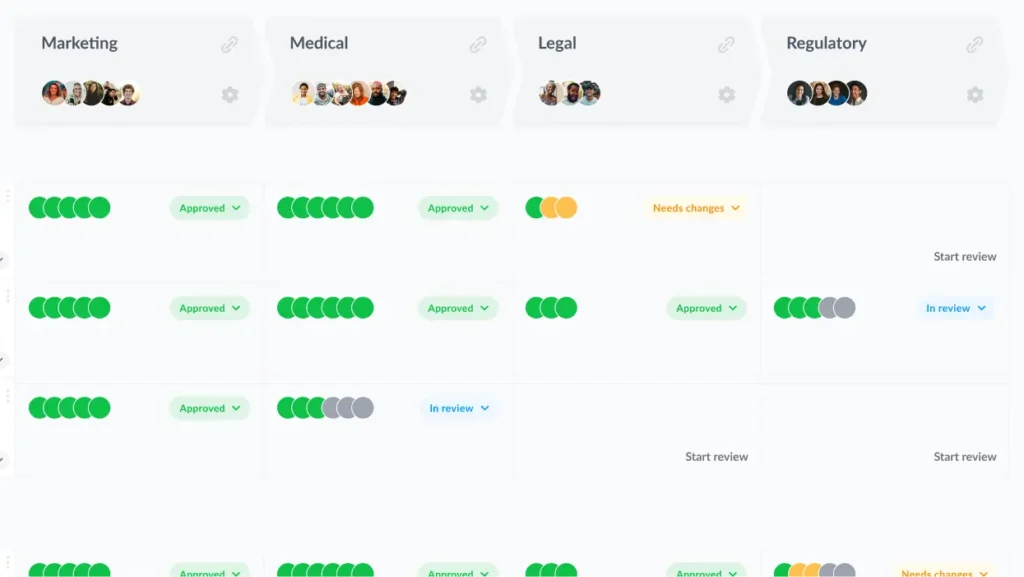
- Comparing versions to make sure no errors or edits have been missed
A robust online proofing platform like Filestage can shave as much as 30% off your review process, so you can get your new medicine packaging to market faster and with fewer compliance holdups.
4. Measure your performance and look for opportunities to improve
Once your new and improved pharmaceutical promotional review process is up and running, it’s time to measure its success. This is the best way to gauge how effective your updated workflows are.
Some example KPIs to track include:
- How long it takes to complete reviews
- The number of review rounds needed for each piece of content
Analyzing the quality of reviewer comments can also be an eye-opening experience. What percentage of them are actionable and solution-oriented? If the number is high, that’s a good sign your process is working.
Regularly tracking KPIs helps you identify areas for improvement in your workflows and gives you the data you need to make informed decisions about your review processes.
And gathering these insights doesn’t have to be time-consuming.
The Filestage Insights dashboard gives you a quick, 30-day overview of key performance metrics, including:
- Projects created
- File versions shared
- Reviews started
- Approvals and change requests submitted
- Average comments per review
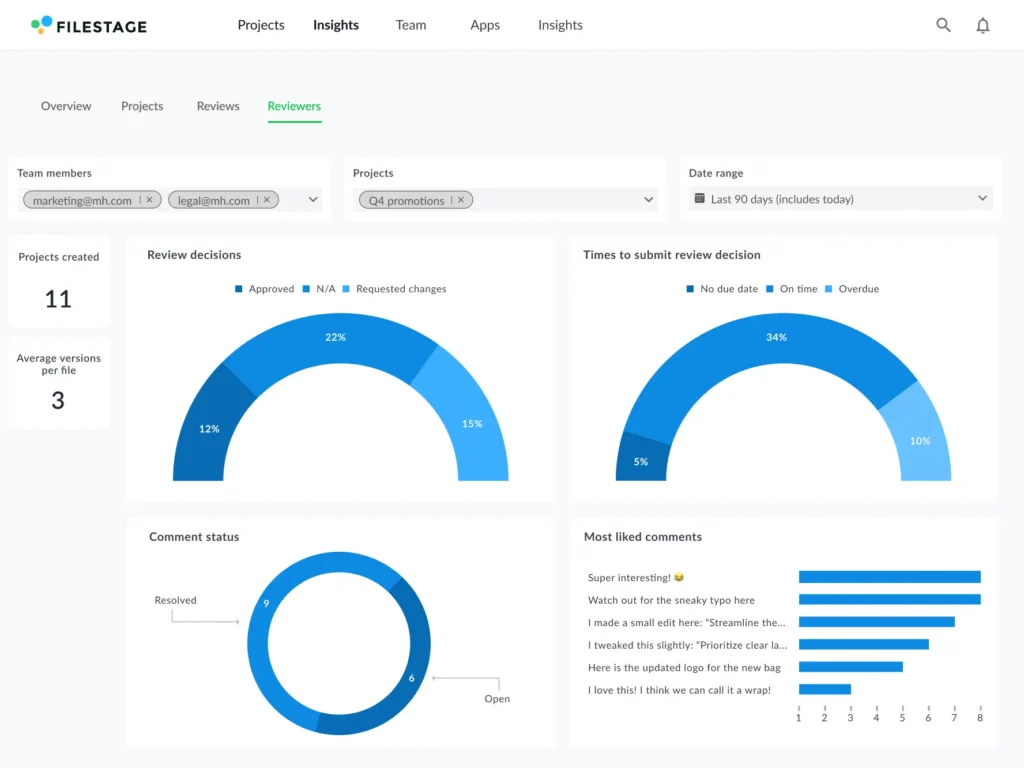
Most importantly, with Insights, you can easily visualize bottlenecks in your feedback process and identify where more support is needed.
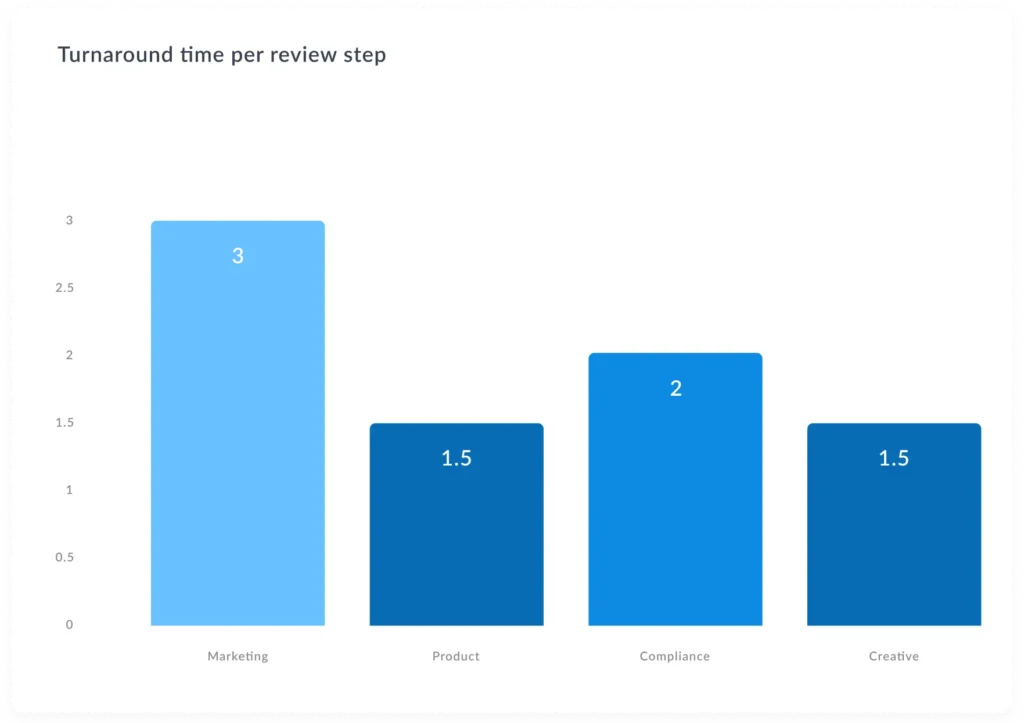
Why does the promotional review process matter in pharma?
In heavily-regulated industries like pharma, every piece of content needs to meet packaging, advertising, and labeling requirements (in line with the FDA or the EU). That includes promotional and advertising materials.
We’re seeing an increase in the number of digital materials and media channels, making the review process more challenging for regulators and also more necessary.
But why is it so important?
Not complying with pharmaceutical regulatory requirements can pose several significant risks for your business:
- Delayed product launches and loss of earnings
- Public health and safety issues
- Damage to your brand reputation
- Legal issues and large fines
And if you’re managing artwork approval in pharma, there’s also a risk of heavy reprinting costs if something slips through the net.
With so much at stake, getting the pharmaceutical promotional review process right is a must.
Optimize pharma content reviews with Filestage
The content review process can be a long and winding road. Multiple reviewer stages, strict compliance regulations, and legal red tape often leave your content at a standstill for weeks. In turn, this causes delays in getting your product to market.
Filestage is designed to support regulated industries in streamlining the content review process so they can get their best work approved faster and with fewer edits. Get a free seven-day trial today and start cutting your review and approval time by as much as 30%.